SYMPOSIUM
2ND INTERNATIONAL SYMPOSIUM 2022

OVERVIEW
Clinical trials are part of the process of developing safe and effective medical treatments and technologies to improve clinical care (1). The clinical trial industry continues to grow as resource limited Countries face a huge burden of diseases. However, the populations of these countries are still under-represented in clinical trials. In order to ensure equity in healthcare and make medicines accessible to all, clinical trial processes must evolve to achieve appropriate representativeness.
Several barriers, including limited human capacity, lack of suitable trial settings, limited funding allocation, delays in regulatory, and ethical review limit the participation of theses underrepresented communities (2).
This online symposium focused on how some ASEAN and African countries have coped with these issues and could establish the environment for the conduct of safe and effective clinical trials. In addition, speakers will share their experience on regional and international collaboration in the conduct of clinical trials.
REFERENCES
1. Graef KM, Okoye I, Ohene Oti NO, Dent J, Odedina FT. Operational Strategies for Clinical Trials in Africa. JCO Glob Oncol. 2020;6:973-82.
2. Franzen SRP, Chandler C, Enquselassie F, Siribaddana S, Atashili J, Angus B, et al. Understanding the investigators: a qualitative study investigating the barriers and enablers to the implementation of local investigator-initiated clinical trials in Ethiopia. BMJ Open. 2013;3(11):e003616.
FORMAT
- This 100% Online symposium involved 16 speakers from 7 countries including Indonesia, Thailand, The Philippines, The Democratic Republic of the Congo, Sudan, USA and Japan.
- Presentations were shared as pre-recorded video from individual speakers and were followed by panel discussions.
- 439 registrants with 259 participants on Day 1 and 325 on Day 2 from various backgrounds including physicians, pharmaceutical company’s delegates, government representatives, etc. joined the symposium.
Table 1. Registrations and participations rates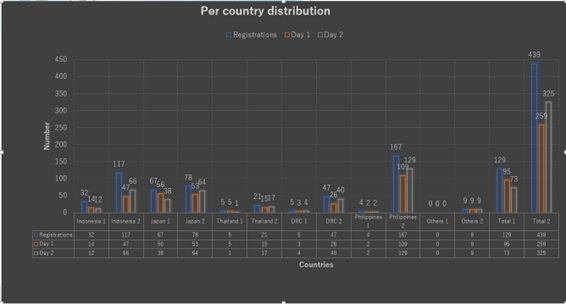
REGISTRANTS’ BACKGROUND
Majority of participants were Medical Doctors (41.5%) and from universities (30.7%). Philippines participants were the most represented during the both days.
Table 2. Participant’s backgrounds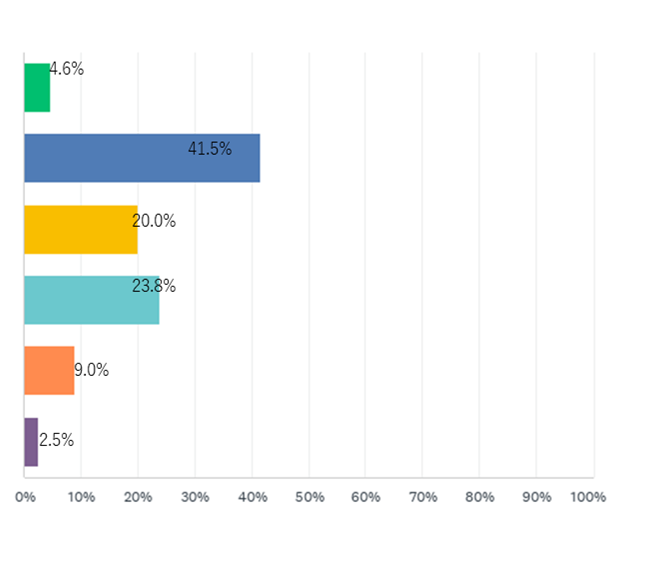
Table 3. Registrations rates per type of organizations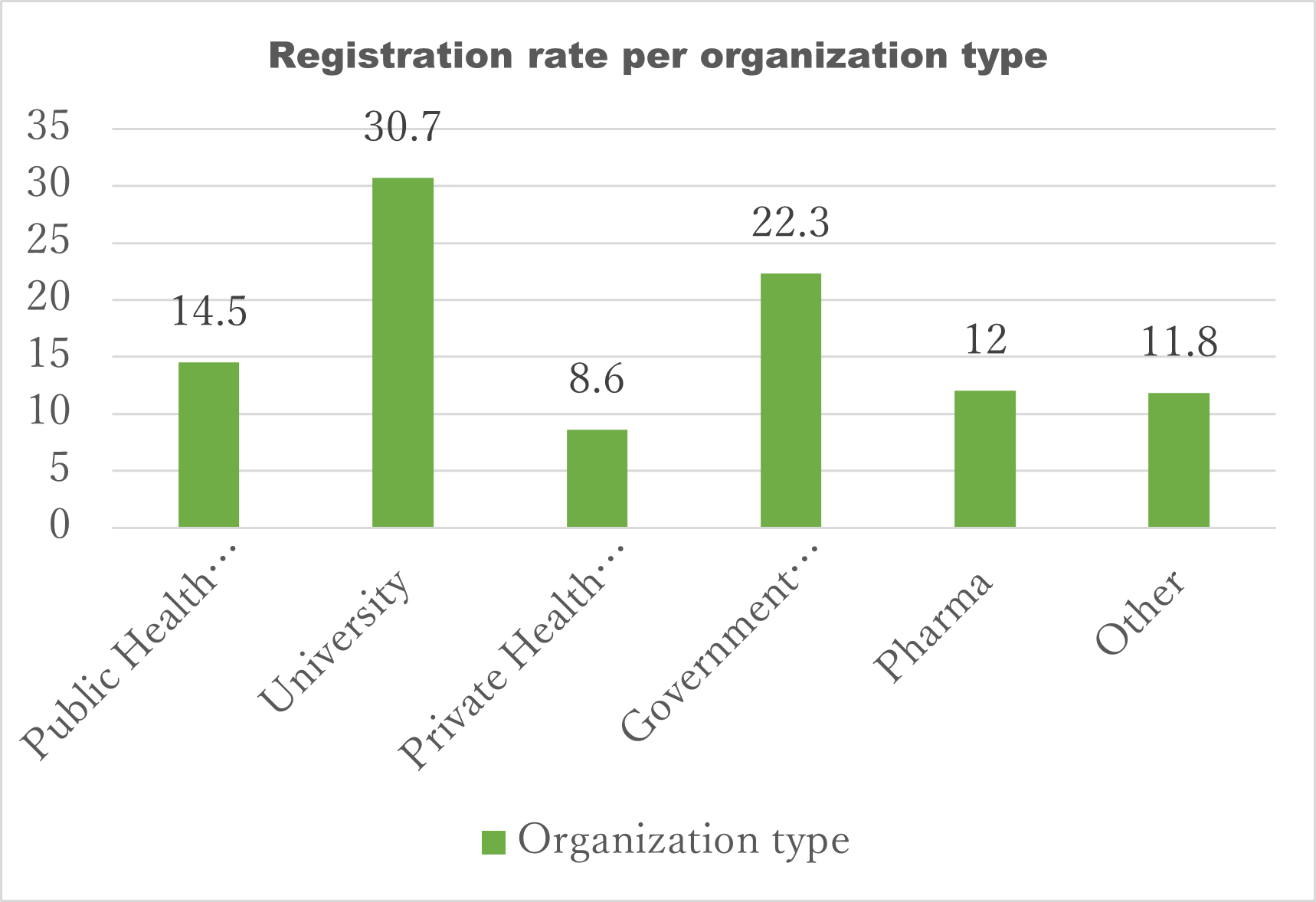
Mar 03-04, 2022
 Flyer
Flyer
"Setting up preclinical and clinical trial in resource-limited contexts: challenges and current state"
INTERNATIONAL SYMPOSIUM (ONLINE) 2021
Mar 11-12, 2021
 List of Abstract
List of Abstract
"International Clinical Trials during COVID-19 era: Should we suspend or continue?"
MRCT SHORT TRAINING PROGRAM (MRCT STP)
MRCT SHORT TRAINING PROGRAM 2020

Jan 18-31, 2020
![]() Booklet 2020
Booklet 2020
"Experimental model for capacity building of clinical research professionals in developing countries."
During 14 days from January 18-31, Researchers delegated from research institutions and hospitals located in six countries, including The Democratic Republic of the Congo (2), Indonesia (3), The Philippines (3), Thailand (3), Vietnam (2) and Japan (1) participated to this multiregional clinical trials immersion program. This is in order to solidify the international network and to improve capacity of clinical research professionals from the five counterpart countries and Japan in the conduct of multiregional clinical research.
Same as the first training, lectures provided by various experts from medicines and medical devices research and development industry, ranging from regulatory authority such as the Pharmaceutical and Medical Devices Agency (PMDA), Academia namely Osaka University, hospitals and research institutions , including the National Cancer Center and the National Center for Global Health and Medicine (NCGM), and pharmaceutical industry namely SHIONOGI & CO., LTD. Lectures were also provided by a delegate from the Japan International Cooperation Agency (JICA) and from the Association of Clinical Research Professionals-Japan chapter (ACRP-J).
In addition to lectures, participants visited two museums in which activities of medical and pharmaceuticals innovations are exposed, including the National Museum of Emerging Science and Innovation: “MIRAIKAN” and Daiichi Sankyo Medicine Museum: “KUSURI MUSEUM”.
MRCT SHORT TRAINING PROGRAM 2019

Jan 16-25, 2019
 Booklet 2019
Booklet 2019
"Comprehensive and collaborative training on medical innovations adapted to challenges of clinical trials in Asian and African countries."
The 10-day program gathered ten researchers from five countries, including the Democratic Republic of the Congo, Indonesia, the Philippines, Thailand, and Vietnam and was held in 2019 from January 16 to 25.
Lectures provided by experts from inside and outside the NCGM, including the Pharmaceutical and Medical Devices Agency (PMDA), Daiichi Sankyo Company, Ltd., the National Cancer Japan, the Graduate School of Public Health, St. Luke's International University and the Institute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University – Waseda University Joint Institution for Advanced Biomedical Sciences (TWIns), emphasized on the role of all stakeholders implicated in the research and development process of medicines and medical devices.
Home country-specific challenges on the conduct of clinical trials were presented by participants during an open seminar in order to share relevant information for the growing network.
“The training was very organized; the topics/lectures/schedule was strictly followed; the lectures were appropriate; the site visits to the other institutions were inspiring; the staff were very friendly and they took care of the group very well; it was a great experience; we all learned a lot from the training.”
Dr. Mylene U. Cornel, St. Luke’s Medical Center, The Philippines
SAKURA SCIENCE PLAN (SSP)
Funded by the Japan Science and Technology Agency (JST), the Sakura Science Plan is a training program which aims to introduce and offer to collaborative countries’ researchers and professionals the experience in Japanese science and technology.
SAKURA SCIENCE PLAN 2018

Jan 16-24, 2018
"Latest topics in the field of Infectious Diseases for Japanese strategic development of future medicine through global research collaboration."
In 2018 from January 16 to 24, eight health professionals, including medical doctors, nurses, and a biostatistics expert, were invited from four medical institutions of four countries, namely: Indonesia, the Philippines, Thailand and Vietnam. Aiming to develop a foundation for a multi-regional clinical research system in the region, the training was designed to improve capacity of the specialists to take a leading role in their countries. Lectures and facility visits were provided and lead by specialists inside and outside of NCGM, focusing on gaining a broad understanding of the Japanese medical and health care system, and the need for multi-regional clinical research. In the second half of this training, participants presented their ideas on “The Need for Clinical Research of Infectious Diseases in Asia” where the discussion demonstrated every participant’s strong commitment and understanding towards the prospects and challenges of the task they will take part in.
“The program Sakura Science have provided information about research and clinical trial. Have more relation from the other country.”
Dr. Atika Rahmawani, RSPI Prof. Sulianti Saroso Hospital, Indonesia
SAKURA SCIENCE PLAN 2016

Nov 22-30, 2016
"Latest topics in the field of Infectious Diseases for Japanese strategic development of future medicine through global research collaboration."
The 2016 Sakura Science Plan, held from November 22 to 30, engaged six doctors from three medical institutions of Indonesia and Vietnam. This training focused on establishing a collaborative network for a reliable system in the region that aims to conduct medical innovation activities. Through various lectures and discussions, the outline of the Japanese health care system was introduced as well as NCGM’s history for decades of contribution to the international health cooperation field and its leading role in response to infectious diseases in Japan and abroad. Visits to institutions inside and out of NCGM provided opportunities to gain insight into Japan’s research and development activities. This training, which consisted of active discussions and sharing of ideas and opinions, successfully brought a strong relationship among the institutions for initiating the collaborative network.



