TARGET MEDICAL PRODUCTS
Japan has advanced in developing a wide array of quality medical products that might have greater benefits when introduced to the global community. However, many of these products and their effectiveness are only recognized in Japan.
For example, because of the low prevalence of malaria, dengue, and/or hepatitis B in Japan, development strategy of devices, drugs, or vaccines for such diseases may have been given less focus. But could otherwise bring major diagnostic and therapeutic improvement when introduced in countries highly afflicted with the same diseases.
DIT supports to secure licenses overseas for these medical innovations: drugs, vaccines, IVD, medical devices developed in Japan.
DIT also supports to acquire necessary clinical evidences by clinical researches to upgrade the clinical procedures, so-called Evidence-based Medicine (EBM).
DRUG REPURPOSING
Drugs approved in Japan for a particular disease can have potential use in other diseases or illnesses. To gather enough supporting evidence for this purpose and regulatory approval, clinical investigative studies are necessary.
OVERSEAS REGULATORY APPROVAL
Countries have different regulatory requirements for the distribution of medical products. In such cases, a bridging study or additional clinical studies, considering ethnic factors, are required to provide sufficient evidence.
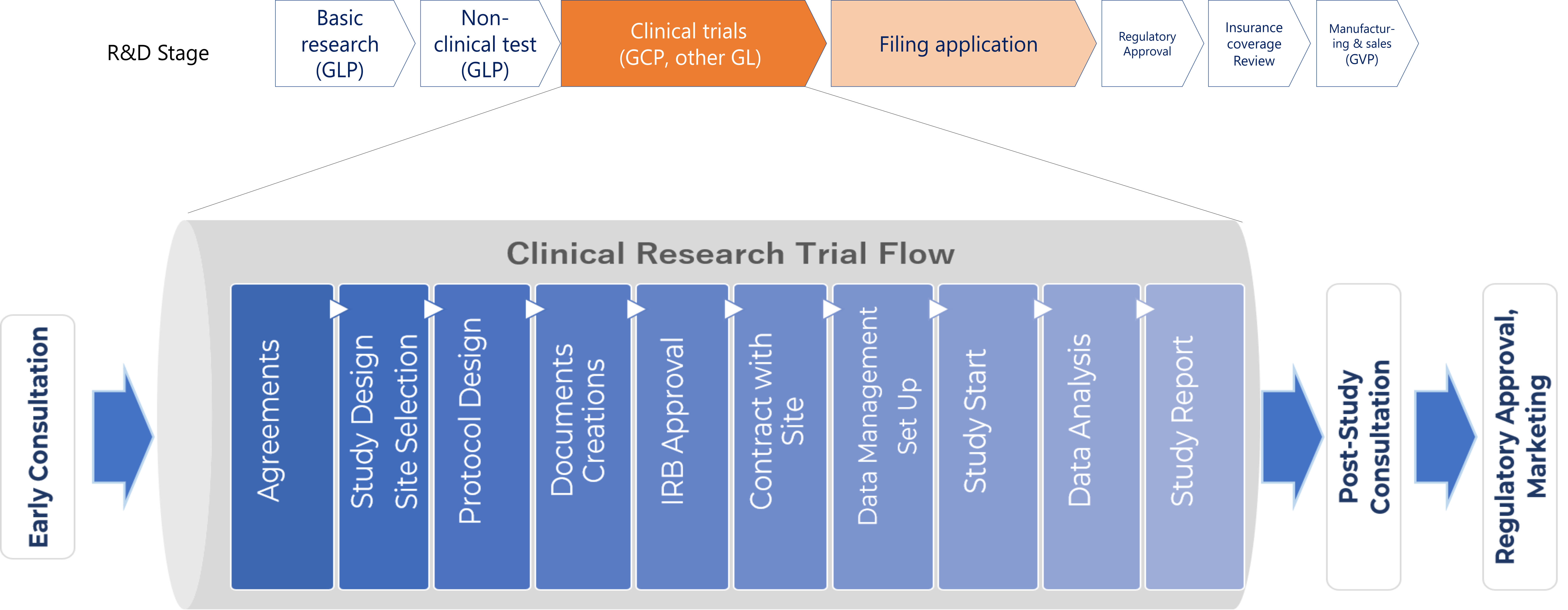
STUDY FLOW
Pipeline flow showing how international clinical research/trial implementation supports business entities:
After feasibility evaluation of the target product for overseas expansion and clinical research/trials through proper consultations, a contract with JIHS/DIT is concluded to assist the implementation.
All phases of the clinical research/trial are supported— research protocol design, selection of test facilities, management and report of test results. DIT also provides business information support related to a product approved to operate in the country.