TYPES OF CLINICAL RESEARCH SETTINGS
Globally there are unmet needs for quality medical products particularly in Low Middle Income Countries (LMICs). In order to bring new innovations to meet these needs, evidence of clinical safety and efficacy must be provided. We facilitate harmonisation and communication between seeds providers and countries with needs by developing different schemes of clinical research such as:
- Multi national clinical trials for emergency response - Covid-19 case
- Multi site clinical research - Drug resistance case in Vietnam
- Joint research case - IVD for malaria case in Thailand
PROJECT 1 : INTERNATIONAL STUDY FOR THE EVALUATION OF SAFETY AND EFFICACY OF ANTIVIRAL FOR COVID-19 TREATMENT
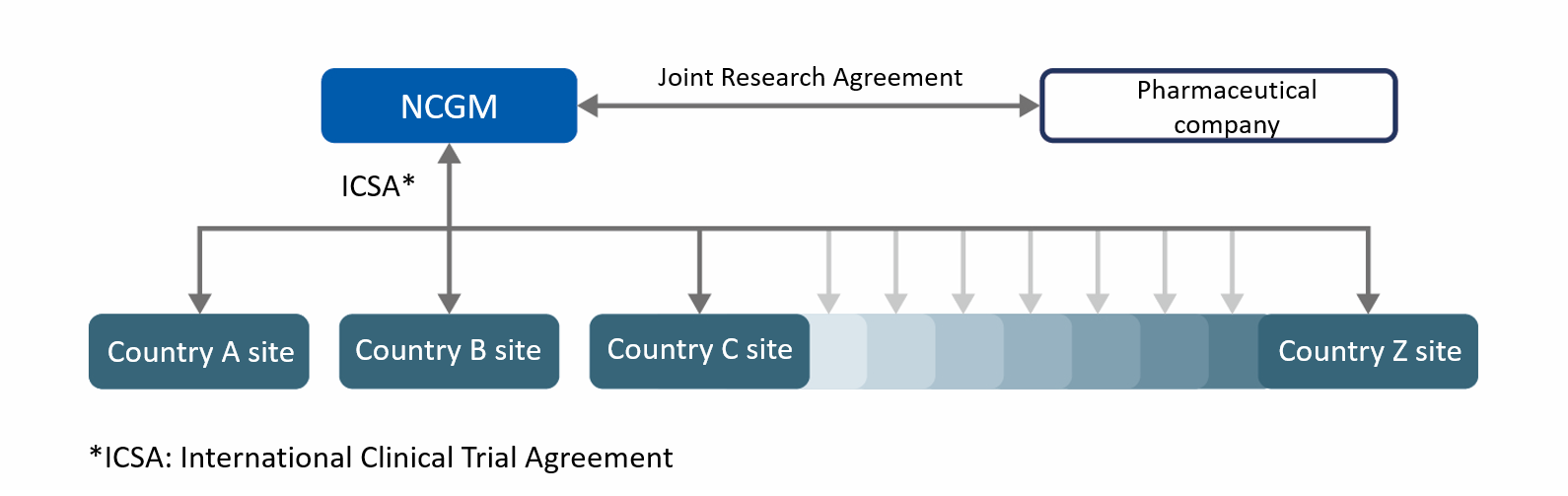
No treatment has yet been established for the novel Coronavirus Disease (COVID-19), which was first reported in China at the end of 2019. In order to respond to pandemics such as COVID-19, clinical use of drugs whose efficacy and safety has not been formally evidenced is required.
To support Japanese government initiatives to distributing antiviral to countries that are affected by the COVID-19, Japan Institute for Health Security has decided to disclose a study protocol with the aim of providing robust evidence and contribute to establishing the appropriate use of the drug for the disease by collaborating with other medical institutions and representative facilities of the afflicted countries.
The project started from middle of May 2020 and expected to end by 2023.
PROJECT 2 : DRUG SUSCEPTIBILITY (MIC) MEASUREMENT OF GRAM-NEGATIVE BACTERIA

Antimicrobial resistance (AMR) is a global threat. Therefore, AMR control is an urgent and important mission for every country. However, it is a difficult task for low- and middle- income countries (LMICs) where the resources are limited. We initiated a collaboration between academia, public and private institutions to bring solutions to AMR problem in Vietnam. A nationwide surveillance was carried out together with Sumitomo Dainippon Pharma and medical institutions in the country. The surveillance was aimed to describe the prevalence and the distribution of four Gram-negative bacteria that are known as the cause for the country's most serious infections. The results of the study will support the management of serious infections and promote the proper use of antibiotics.
【Press Release】JIHS and Sumitomo Dainippon Pharma Initiate International Project on Measures against AMR and Promote the Proper Use of Antibiotics in Vietnam
PROJECT 3 : INTERNATIONAL COLLABORATIVE RESEARCH AND ITS INTERNATIONAL DEPLOYMENT THROUGH THE WHO SCHEME

In Japan, pharmaceutical approval was obtained in May 2020; it is expected to be effectively used in circumstances in which conducting PCR tests is difficult (e.g. medical care system, emergency). To further expand its application internationally we observed the overseas pharmaceutical regulations and WHO scheme for emergency use listing (EUL).
In this project we aim to accumulate clinical evidence regarding the study population, efficacy, safety, clinical pharmacological, and characteristics of each formulation; examination of efficacy and use in actual clinical practice; and pursue international deployment by acquiring pharmaceutical approval in Asian nations and WHO EUL scheme.
PROJECT 4 : NEW DIAGNOSTIC KIT FOR MALARIA IN THAILAND
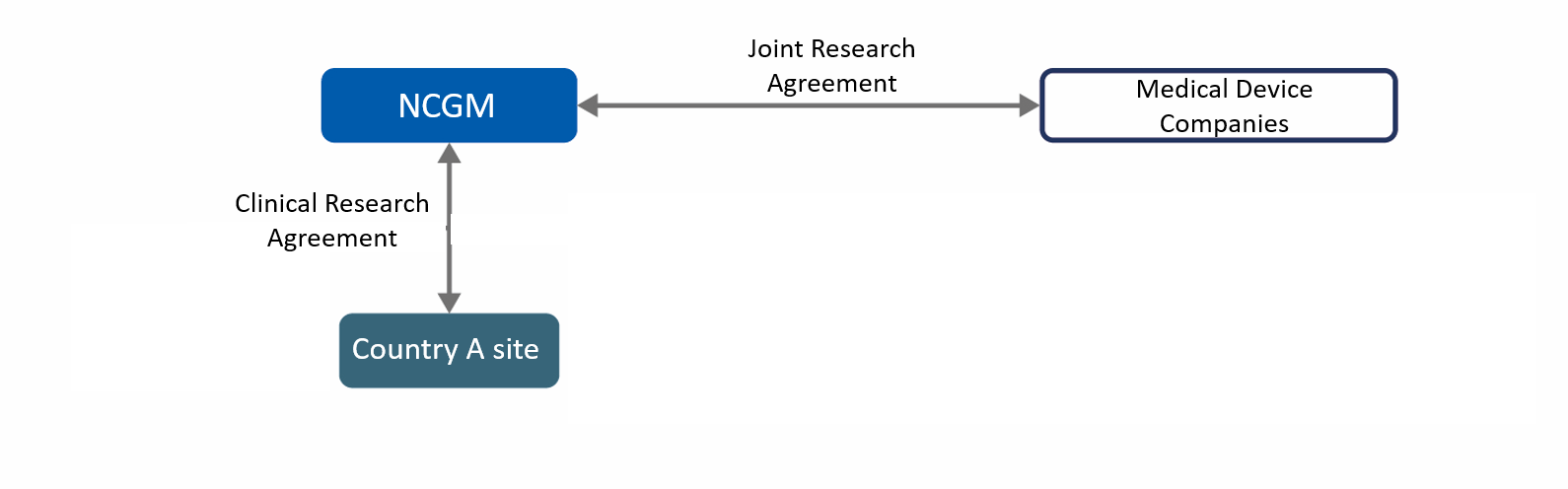
Malaria is one of three major infectious diseases, which affects/kills 220 million and 435 thousand people per year (2017 data), respectively. While Rapid Diagnostic Testing (RDT), microscopic and PCR are currently used for diagnosis as the global standards, each has pros/cons from the aspects of reliability, variance of sensitivity, ease-of-use and cost, which leaves room for improvement. Initiated by the Department of Tropical Medicine and Malaria in JIHS’s Research Institute, this project aims to develop rapid, precise and easy-to-use malaria diagnostics through evaluating two novel medical devices*, which leads to contributions to Global Health.
*Individually developed by EIKEN CHEMICAL CO., LTD. and SYSMEX CORPORATION



